
 Ph.D. candidate, The University of Tokyo
Ph.D. candidate, The University of Tokyo
嗨,欢迎来到我的个人学术主页!
Hi! My name is Cheng Wang (王成, pronounced as "Wahng Churn"). I am currently working as a product development specialist at a leading pharmaceutical company in China. Before that, I graduated from the Institute of Functional Nano & Soft materials (FUNSOM) at Soochow University, where I was supervised by Prof. Qian Chen. My research focuses on biomedical nanomaterials and their applications in cancer therapy and biomedical engineering.
I have been admitted to the Ph.D. program in Bioengineering at The University of Tokyo for Fall 2025 and have been nominated as a candidate for the Japanese Government (Monbu-kagakusho) Scholarship, providing me with a valuable window to further immerse myself in research.
Warning
Problem: The current name of your GitHub Pages repository ("Solution: Please consider renaming the repository to "
http://".
However, if the current repository name is intended, you can ignore this message by removing "{% include widgets/debug_repo_name.html %}" in index.html.
Action required
Problem: The current root path of this site is "baseurl ("_config.yml.
Solution: Please set the
baseurl in _config.yml to "Education
-
 The University of TokyoDepartment of bioengineering
The University of TokyoDepartment of bioengineering
Incoming Ph.D. studentOct. 2025 - Oct. 2028 -
 Soochow UniversityInstitue of Functional Nano & Soft Material (FUNSOM)
Soochow UniversityInstitue of Functional Nano & Soft Material (FUNSOM)
M.E. in Biomedical NanomaterialSep. 2021 - Jul. 2024 -
 Nanjing Tech UniversityB.E. in Polymer MaterialSep. 2017 - Jul. 2021
Nanjing Tech UniversityB.E. in Polymer MaterialSep. 2017 - Jul. 2021
Honors & Awards
-
Soochow University Graduate Academic Scholarship (3 times)2022-2024
-
Honorable Mention in Mathematical Contest In Modeling2020
-
China National Encouragement Scholarship2021
-
First Prize Scholarship of Nanjing Tech University (3 times)2018-2021
News
Selected Publications
(view all )

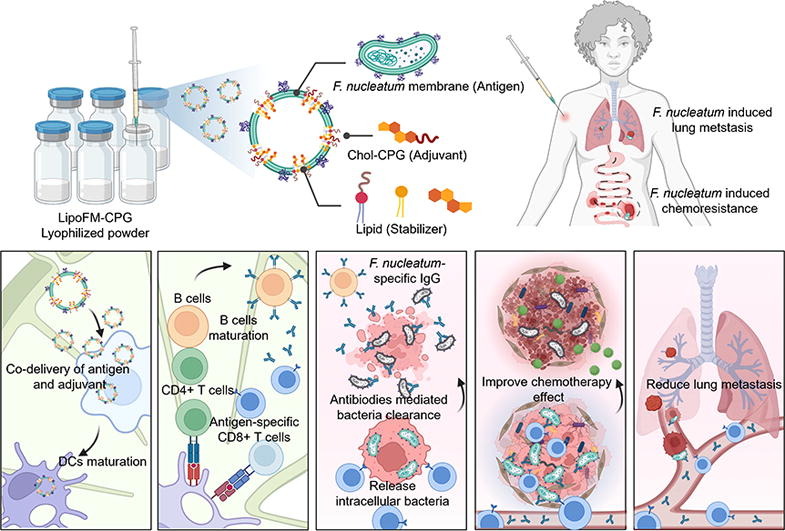
An emerging antibacterial nanovaccine for enhanced chemotherapy by selectively eliminating tumor-colonizing bacteria
Linfu Chen, Zheyu Kang, Jingjing Shen, Rui Zhao, Yu Miao, Lin Zhang, Zixuan Zheng, Zhemin Zhang, Nanhui Liu, Cheng Wang, Huapan Fang, Jun Zhou, Yudong Wang, Zhuang Liu, Yang Yang, Qian Chen* (* corresponding author)
Science Bulletin 2024 Q1IF=18.8
Fusobacterium nucleatum (F. nucleatum), an oral anaerobe, is prevalent in colorectal cancer and is closely related to increased cancer cell growth, metastasis, and poor treatment outcomes. Bacterial vaccines capable of selectively eliminating bacteria present a promising approach to targeting intratumor F. nucleatum, thereby enhancing cancer treatment. Although adjuvants have been employed to enhance the immune response, the vaccine’s effectiveness is constrained by inadequate T-cell activation necessary for eradicating intracellular pathogens. In this study, we developed a minimalistic, biomimetic nanovaccine by integrating highly immunostimulatory adjuvant cholesterol-modified CpG oligonucleotides into the autologously derived F. nucleatum membranes. Compared to the traditional vaccines consisting of inactivated bacteria and Alum adjuvant, the nanovaccine coupled with bacterial membranes and adjuvants could remarkably improve multiple antigens and adjuvant co-delivery to dendritic cells, maximizing their ability to achieve effective antigen presentation and strong downstream immune progress. Notably, the nanovaccine exhibits outstanding selective prophylactic and therapeutic effects, eliminating F. nucleatum without affecting intratumoral and gut microbiota. It significantly enhances chemotherapy efficacy and reduces cancer metastasis in F. nucleatum-infected colorectal cancer. Overall, this work represents the rational application of bacterial nanovaccine and provides a blueprint for future development in enhancing the antitumor effect against bacterial-infected cancer.
An emerging antibacterial nanovaccine for enhanced chemotherapy by selectively eliminating tumor-colonizing bacteria
Linfu Chen, Zheyu Kang, Jingjing Shen, Rui Zhao, Yu Miao, Lin Zhang, Zixuan Zheng, Zhemin Zhang, Nanhui Liu, Cheng Wang, Huapan Fang, Jun Zhou, Yudong Wang, Zhuang Liu, Yang Yang, Qian Chen* (* corresponding author)
Science Bulletin 2024 Q1IF=18.8
Fusobacterium nucleatum (F. nucleatum), an oral anaerobe, is prevalent in colorectal cancer and is closely related to increased cancer cell growth, metastasis, and poor treatment outcomes. Bacterial vaccines capable of selectively eliminating bacteria present a promising approach to targeting intratumor F. nucleatum, thereby enhancing cancer treatment. Although adjuvants have been employed to enhance the immune response, the vaccine’s effectiveness is constrained by inadequate T-cell activation necessary for eradicating intracellular pathogens. In this study, we developed a minimalistic, biomimetic nanovaccine by integrating highly immunostimulatory adjuvant cholesterol-modified CpG oligonucleotides into the autologously derived F. nucleatum membranes. Compared to the traditional vaccines consisting of inactivated bacteria and Alum adjuvant, the nanovaccine coupled with bacterial membranes and adjuvants could remarkably improve multiple antigens and adjuvant co-delivery to dendritic cells, maximizing their ability to achieve effective antigen presentation and strong downstream immune progress. Notably, the nanovaccine exhibits outstanding selective prophylactic and therapeutic effects, eliminating F. nucleatum without affecting intratumoral and gut microbiota. It significantly enhances chemotherapy efficacy and reduces cancer metastasis in F. nucleatum-infected colorectal cancer. Overall, this work represents the rational application of bacterial nanovaccine and provides a blueprint for future development in enhancing the antitumor effect against bacterial-infected cancer.
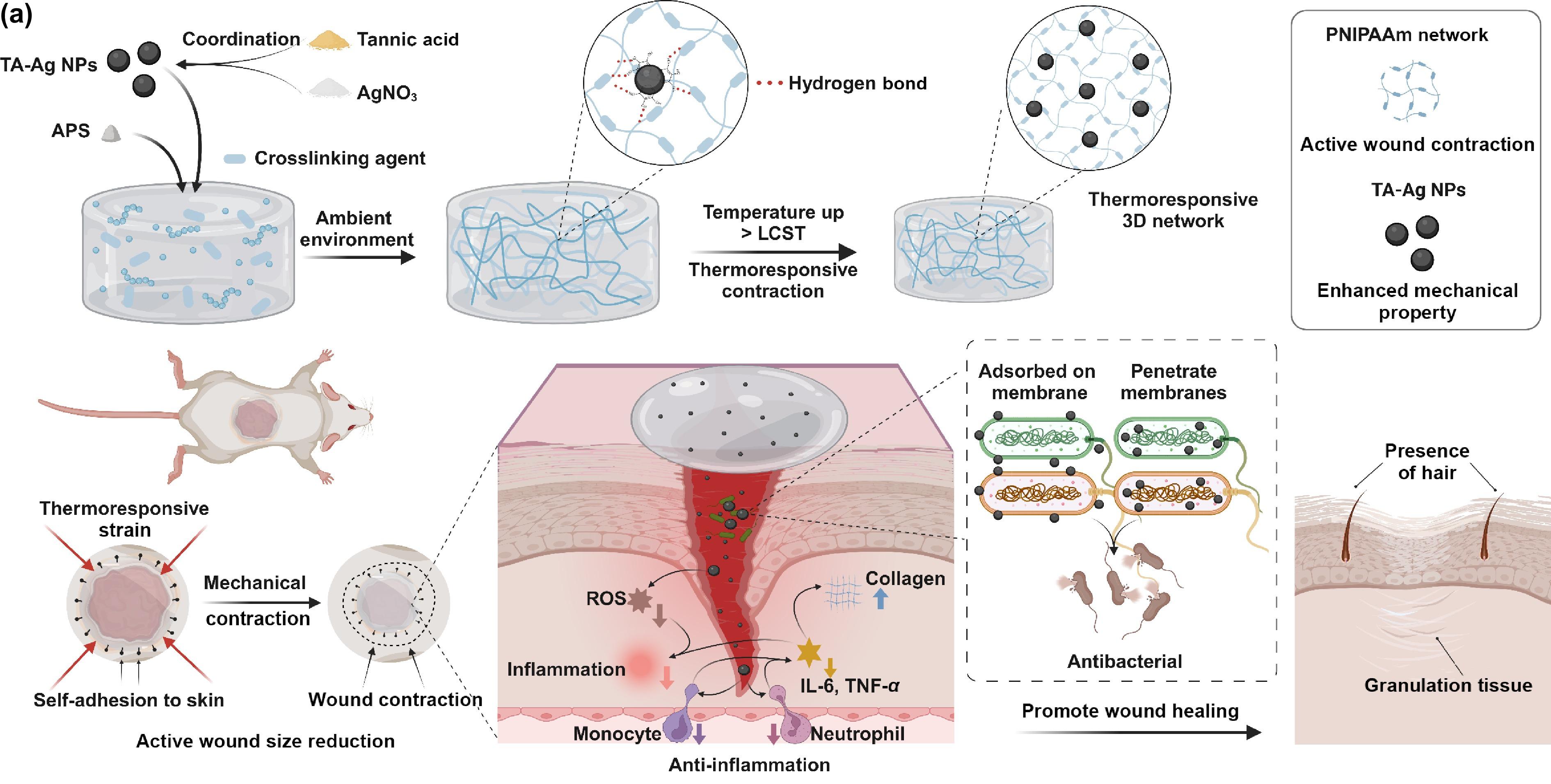
Actively contractible and antibacterial hydrogel for accelerated wound healing
Zhaoxin Ji#, Ting Wei#, Jiafei Zhu, Jiaying Hu, Zhisheng Xiao, Boxiong Bai, Xinying Lv, Yu Miao, Muchao Chen, Cheng Wang, Feng Pan, Yang Yang, Meng Li*, Qian Chen* (# equal contribution, * corresponding author)
Nano Research 2024 Q1IF=9.5
Adhesive hydrogel has drawn great attention for wide applications in wound healing owing to its excellent biocompatibility and lasting adhesiveness. However, traditional adhesive hydrogels only keep the wound moist to promote wound healing. It is still imperative to fabricate adhesive hydrogels that exhibit efficient antibacterial ability, active driving dynamic wound closure, and reactive oxygen species (ROS) scavenging together with excellent mechanical properties. Here, a novel hydrogel based on poly(N-isopropyl acrylamide) (PNIPAAm), a thermoresponsive polymer, and tannic acid (TA)-Ag nanoparticles (TA-Ag NPs) exhibiting active contraction, tissue adhesion, anti-inflammatory and antibacterial functions was developed. TA-Ag dispersed in the hydrogel not only functioned as the catalyst to polymerize the reaction but also provided additional anti-inflammatory and antibacterial properties. Besides, tannic acid containing catechol groups endowed the hydrogel with adhesive ability. More interestingly, the obtained hydrogel exhibited the thermoresponsive shrinkage ability, which could mechanically drive wound closure due to the presence of PNIPAAm network. In vivo mouse full-thickness skin defect model demonstrated that this actively contractible and antibacterial hydrogel is a promising dressing to improve wound healing process by accelerating tissue regeneration and preventing bacterial infection. Therefore, this multi-functional adhesive hydrogel developed here may provide a new possibility for wound healing.
Actively contractible and antibacterial hydrogel for accelerated wound healing
Zhaoxin Ji#, Ting Wei#, Jiafei Zhu, Jiaying Hu, Zhisheng Xiao, Boxiong Bai, Xinying Lv, Yu Miao, Muchao Chen, Cheng Wang, Feng Pan, Yang Yang, Meng Li*, Qian Chen* (# equal contribution, * corresponding author)
Nano Research 2024 Q1IF=9.5
Adhesive hydrogel has drawn great attention for wide applications in wound healing owing to its excellent biocompatibility and lasting adhesiveness. However, traditional adhesive hydrogels only keep the wound moist to promote wound healing. It is still imperative to fabricate adhesive hydrogels that exhibit efficient antibacterial ability, active driving dynamic wound closure, and reactive oxygen species (ROS) scavenging together with excellent mechanical properties. Here, a novel hydrogel based on poly(N-isopropyl acrylamide) (PNIPAAm), a thermoresponsive polymer, and tannic acid (TA)-Ag nanoparticles (TA-Ag NPs) exhibiting active contraction, tissue adhesion, anti-inflammatory and antibacterial functions was developed. TA-Ag dispersed in the hydrogel not only functioned as the catalyst to polymerize the reaction but also provided additional anti-inflammatory and antibacterial properties. Besides, tannic acid containing catechol groups endowed the hydrogel with adhesive ability. More interestingly, the obtained hydrogel exhibited the thermoresponsive shrinkage ability, which could mechanically drive wound closure due to the presence of PNIPAAm network. In vivo mouse full-thickness skin defect model demonstrated that this actively contractible and antibacterial hydrogel is a promising dressing to improve wound healing process by accelerating tissue regeneration and preventing bacterial infection. Therefore, this multi-functional adhesive hydrogel developed here may provide a new possibility for wound healing.
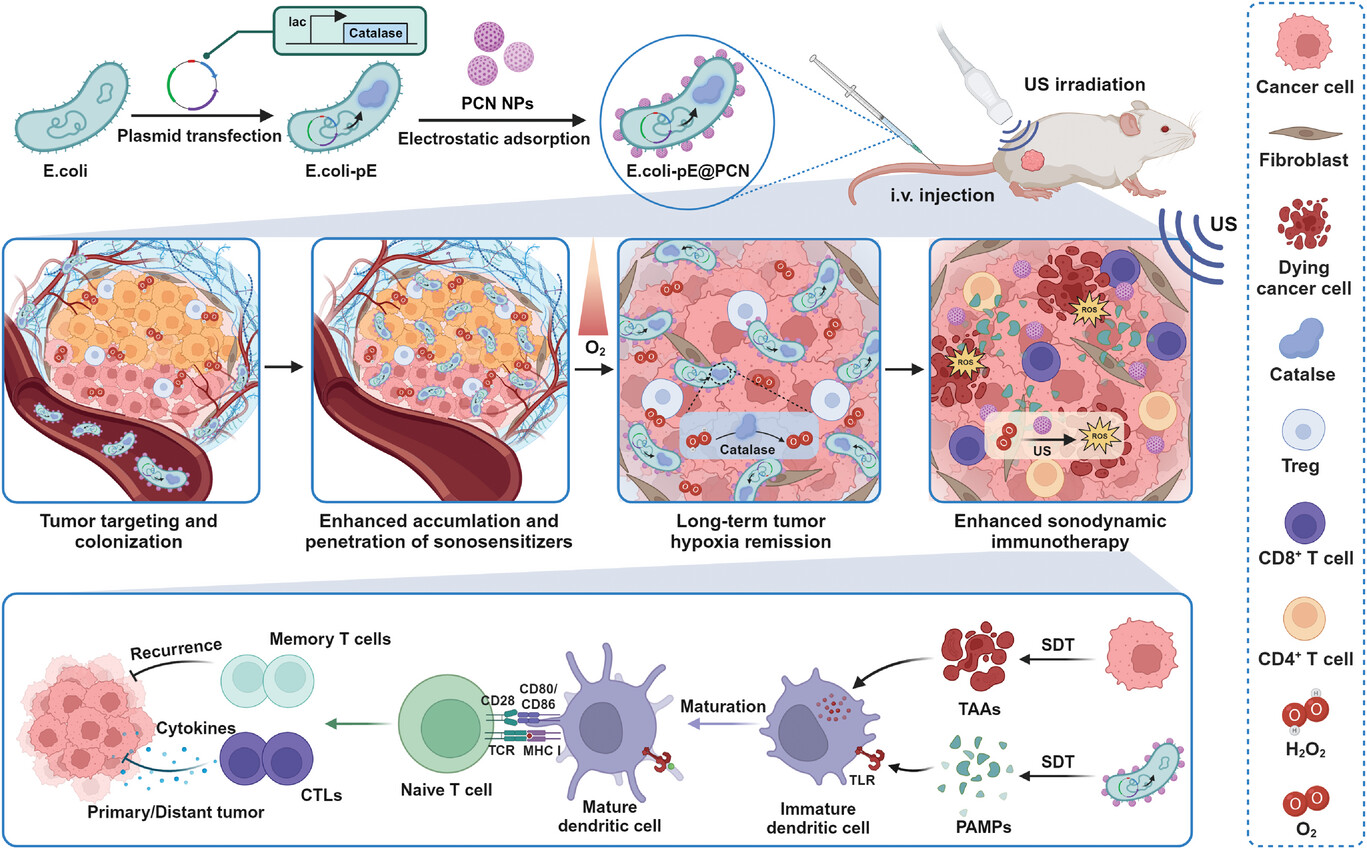
Programmable Bacteria-Based Biohybrids as Living Biotherapeutics for Enhanced Cancer Sonodynamic-Immunotherapy
Cheng Wang, Linfu Chen, Jiafei Zhu, Chunjie Wang, Maoyi Li, Yu Miao, Nanhui Liu, Zhaoxin Ji, Feng Pan, Yi Liu, Junjie Zhu, Yang Yang, Qian Chen* (* corresponding author)
Advanced Functional Materias 2024 Q1IF=18.5
Synthetic biology is propelling medicine into a new era through its capacity to genetically program living cells. One of the particular interests is engineering bacteria as a live and targeted therapeutic delivery system. Herein, the bacterial biohybrid (E. coli-pE@PCN) is developed by genetically engineering Escherichia coli BL21 to overexpress catalase (E. coli-pE) and electrostatically adsorbing nano-sonosensitizers (PCN NPs) for enhanced and targeted sonodynamic therapy (SDT). Leveraging the ability to colonize and penetrate deep in tumors, engineered bacteria can not only sustainably express catalase to relieve tumor hypoxia, but also facilitate the enriched and expanded distribution of the carried sonosensitizer at the tumor site, so as to trigger effective SDT. More interestingly, it is found that E. coli-pE@PCN-based SDT can successfully inhibit the growth of subcutaneous and orthotopic colorectal tumors by inducing potent antitumor immune responses due to the released tumor-associated antigens and native immunogenicity of bacterial pathogen-associated molecular patterns. Furthermore, E. coli-pE@PCN-based SDT can not only prime a strong immune memory response to prevent tumor recurrence but also elicit a potent abscopal effect to inhibit tumor metastasis. Therefore, the programmable bacteria-based biohybrids developed here pave an avenue to prepare next-generation sonodynamic-immunotherapeutics to eliminate cancer and prevent its relapse and metastasis.
Programmable Bacteria-Based Biohybrids as Living Biotherapeutics for Enhanced Cancer Sonodynamic-Immunotherapy
Cheng Wang, Linfu Chen, Jiafei Zhu, Chunjie Wang, Maoyi Li, Yu Miao, Nanhui Liu, Zhaoxin Ji, Feng Pan, Yi Liu, Junjie Zhu, Yang Yang, Qian Chen* (* corresponding author)
Advanced Functional Materias 2024 Q1IF=18.5
Synthetic biology is propelling medicine into a new era through its capacity to genetically program living cells. One of the particular interests is engineering bacteria as a live and targeted therapeutic delivery system. Herein, the bacterial biohybrid (E. coli-pE@PCN) is developed by genetically engineering Escherichia coli BL21 to overexpress catalase (E. coli-pE) and electrostatically adsorbing nano-sonosensitizers (PCN NPs) for enhanced and targeted sonodynamic therapy (SDT). Leveraging the ability to colonize and penetrate deep in tumors, engineered bacteria can not only sustainably express catalase to relieve tumor hypoxia, but also facilitate the enriched and expanded distribution of the carried sonosensitizer at the tumor site, so as to trigger effective SDT. More interestingly, it is found that E. coli-pE@PCN-based SDT can successfully inhibit the growth of subcutaneous and orthotopic colorectal tumors by inducing potent antitumor immune responses due to the released tumor-associated antigens and native immunogenicity of bacterial pathogen-associated molecular patterns. Furthermore, E. coli-pE@PCN-based SDT can not only prime a strong immune memory response to prevent tumor recurrence but also elicit a potent abscopal effect to inhibit tumor metastasis. Therefore, the programmable bacteria-based biohybrids developed here pave an avenue to prepare next-generation sonodynamic-immunotherapeutics to eliminate cancer and prevent its relapse and metastasis.
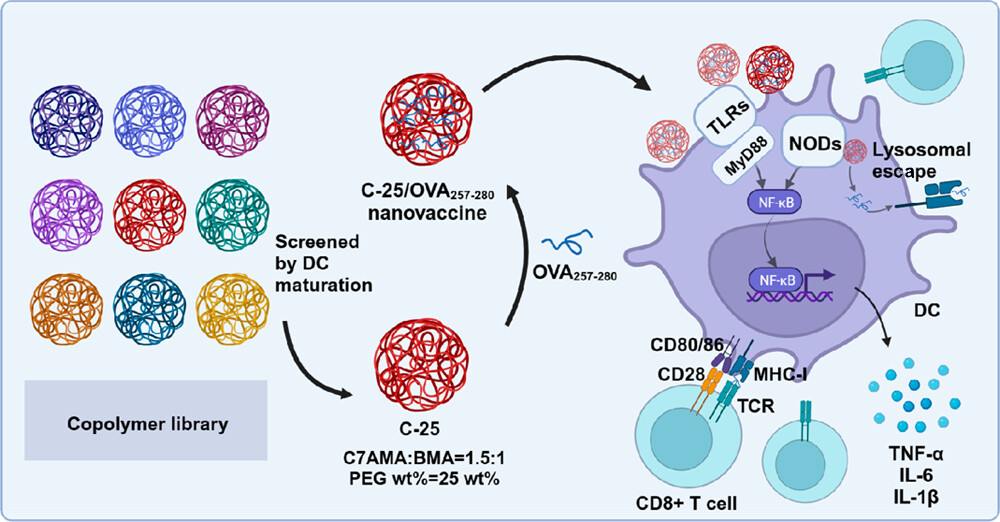
Minimalist Nanovaccine with Optimized Amphiphilic Copolymers for Cancer Immunotherapy
Le Niu#, Yu Miao#, Zhiqin Cao, Ting Wei, Jiafei Zhu, Maoyi Li, Boxiong Bai, Linfu Chen, Nanhui Liu, Feng Pan, Junjie Zhu, Cheng Wang, Yang Yang, Qian Chen* (# equal contribution, * corresponding author)
ACS Nano 2024 Q1IF=15.8
Cancer vaccines with the ability to elicit tumor-specific immune responses have attracted significant interest in cancer immunotherapy. A key challenge for effective cancer vaccines is the spatiotemporal codelivery of antigens and adjuvants. Herein, we synthesized a copolymer library containing nine poly(ethylene glycol) methyl ether methacrylate-co-butyl methacrylate-co-2-(azepan-1-yl)ethyl methacrylate (PEGMA-co-BMA-co-C7AMA) graft copolymers with designed proportions of different components to regulate their properties. Among these polymers, C-25, with a C7AMA:BMA ratio at 1.5:1 and PEG wt % of 25%, was screened as the most effective nanovaccine carrier with enhanced ability to induce mouse bone marrow-derived dendritic cell (BMDC) maturation. Additionally, RNA-sequencing (RNA-Seq) analysis revealed that C-25 could activate dendritic cells (DCs) through multisignaling pathways to trigger potent immune effects. Then, the screened C-25 was used to encapsulate the model peptide antigen, OVA257-280, to form nanovaccine C-25/OVA257-280. It was found that the C-25/OVA257-280 nanovaccine could effectively facilitate DC maturation and antigen cross-presentation without any other additional adjuvant and exhibited excellent prophylactic efficacy in the B16F10-OVA tumor model. Moreover, in combination with antiprogrammed cell death protein-ligand 1 (anti-PD-L1), the C-25/OVA257-280 nanovaccine could significantly delay the growth of pre-existing tumors. Therefore, this work developed a minimalist nanovaccine with a simple formulation and high efficiency in activating tumor-specific immune responses, showing great potential for further application in cancer immunotherapy.
Minimalist Nanovaccine with Optimized Amphiphilic Copolymers for Cancer Immunotherapy
Le Niu#, Yu Miao#, Zhiqin Cao, Ting Wei, Jiafei Zhu, Maoyi Li, Boxiong Bai, Linfu Chen, Nanhui Liu, Feng Pan, Junjie Zhu, Cheng Wang, Yang Yang, Qian Chen* (# equal contribution, * corresponding author)
ACS Nano 2024 Q1IF=15.8
Cancer vaccines with the ability to elicit tumor-specific immune responses have attracted significant interest in cancer immunotherapy. A key challenge for effective cancer vaccines is the spatiotemporal codelivery of antigens and adjuvants. Herein, we synthesized a copolymer library containing nine poly(ethylene glycol) methyl ether methacrylate-co-butyl methacrylate-co-2-(azepan-1-yl)ethyl methacrylate (PEGMA-co-BMA-co-C7AMA) graft copolymers with designed proportions of different components to regulate their properties. Among these polymers, C-25, with a C7AMA:BMA ratio at 1.5:1 and PEG wt % of 25%, was screened as the most effective nanovaccine carrier with enhanced ability to induce mouse bone marrow-derived dendritic cell (BMDC) maturation. Additionally, RNA-sequencing (RNA-Seq) analysis revealed that C-25 could activate dendritic cells (DCs) through multisignaling pathways to trigger potent immune effects. Then, the screened C-25 was used to encapsulate the model peptide antigen, OVA257-280, to form nanovaccine C-25/OVA257-280. It was found that the C-25/OVA257-280 nanovaccine could effectively facilitate DC maturation and antigen cross-presentation without any other additional adjuvant and exhibited excellent prophylactic efficacy in the B16F10-OVA tumor model. Moreover, in combination with antiprogrammed cell death protein-ligand 1 (anti-PD-L1), the C-25/OVA257-280 nanovaccine could significantly delay the growth of pre-existing tumors. Therefore, this work developed a minimalist nanovaccine with a simple formulation and high efficiency in activating tumor-specific immune responses, showing great potential for further application in cancer immunotherapy.
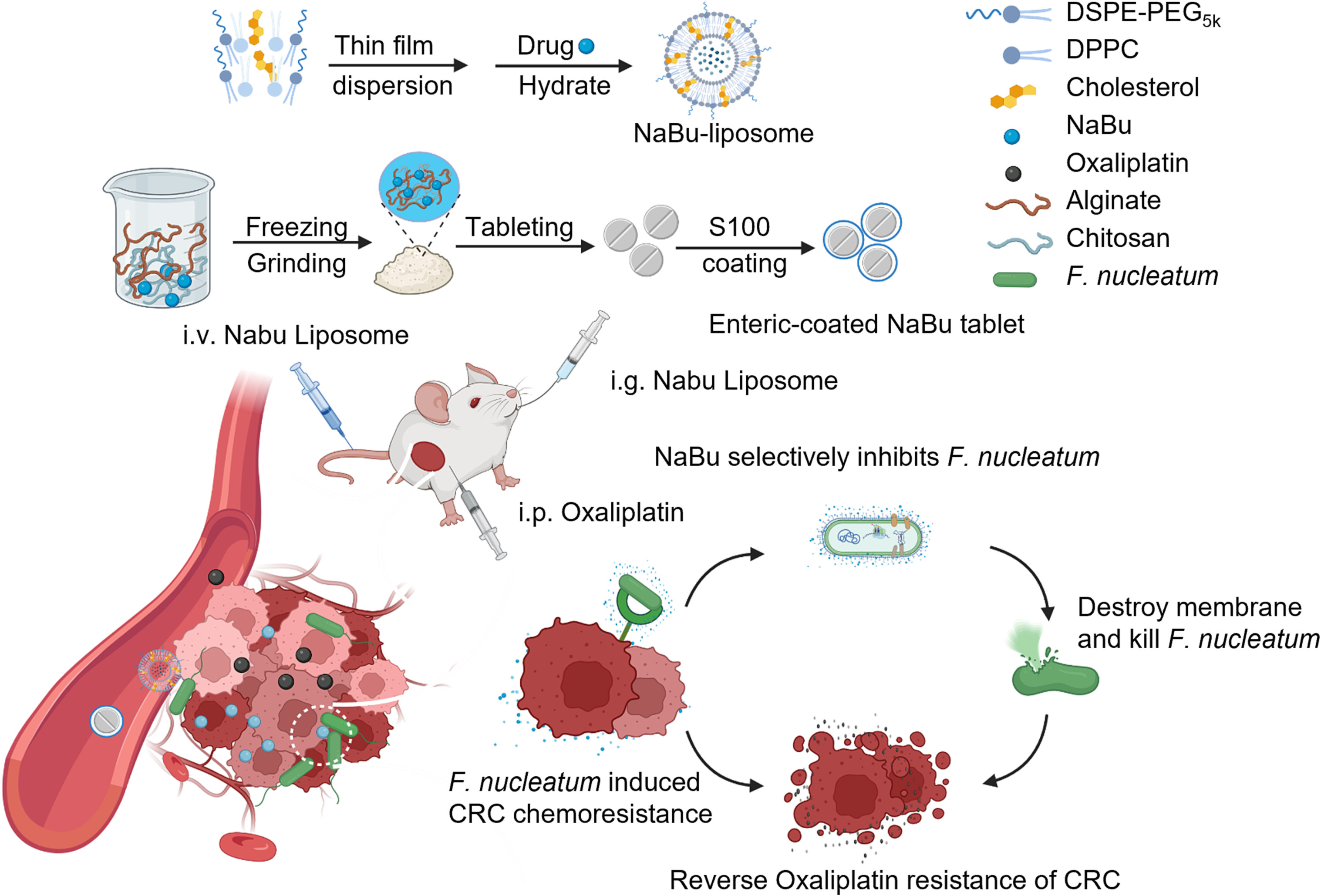
Delivery of short chain fatty acid butyrate to overcome Fusobacterium nucleatum-induced chemoresistance
Linfu Chen#, Rui Zhao#, Zheyu Kang, Zhiqin Cao, Nanhui Liu, Jingjing Shen, Cheng Wang, Feng Pan, Xiao Zhou, Zhuang Liu, Yang Yang, Qian Chen* (# equal contribution, * corresponding author)
Journal of Controlled Release 2023 Q1IF=10.5
The gut microbiota is closely associated with the progression of colorectal cancer (CRC) in which Fusobacterium nucleatum (F. nucleatum) was found to induce cancer resistance to chemotherapeutics. To relieve F. nucleatum induced drug resistance, herein, we found that short-chain fatty acid butyrate can inhibit the growth, enrichment and adhesion of F. nucleatum in colorectal cancer tissues by downregulating the expression of adhesion-associated outer membrane proteins, including RadD, FomA, and FadA, to reduce the colonization and invasion of F. nucleatum and relieve the chemoresistance induced by F. nucleatum. Leveraging the killing effect of butyrate on F. nucleatum, sodium butyrate (NaBu) was encapsulated in liposomes or prepared as NaBu tablets with Eudragit S100 coating and administered by intravenous injection or oral administration, respectively. Interestingly, both intravenous administration of NaBu liposomes and oral delivery of NaBu tablets could effectively inhibit the proliferation of F. nucleatum and significantly improve the therapeutic efficacy of oxaliplatin in mice with subcutaneous colorectal tumors, orthotopic colorectal tumors and even spontaneously formed colorectal tumors. Thus, our work provides a simple but effective formulation of NaBu to relieve F. nucleatum induced chemoresistance, exhibiting ideal clinical application prospects.
Delivery of short chain fatty acid butyrate to overcome Fusobacterium nucleatum-induced chemoresistance
Linfu Chen#, Rui Zhao#, Zheyu Kang, Zhiqin Cao, Nanhui Liu, Jingjing Shen, Cheng Wang, Feng Pan, Xiao Zhou, Zhuang Liu, Yang Yang, Qian Chen* (# equal contribution, * corresponding author)
Journal of Controlled Release 2023 Q1IF=10.5
The gut microbiota is closely associated with the progression of colorectal cancer (CRC) in which Fusobacterium nucleatum (F. nucleatum) was found to induce cancer resistance to chemotherapeutics. To relieve F. nucleatum induced drug resistance, herein, we found that short-chain fatty acid butyrate can inhibit the growth, enrichment and adhesion of F. nucleatum in colorectal cancer tissues by downregulating the expression of adhesion-associated outer membrane proteins, including RadD, FomA, and FadA, to reduce the colonization and invasion of F. nucleatum and relieve the chemoresistance induced by F. nucleatum. Leveraging the killing effect of butyrate on F. nucleatum, sodium butyrate (NaBu) was encapsulated in liposomes or prepared as NaBu tablets with Eudragit S100 coating and administered by intravenous injection or oral administration, respectively. Interestingly, both intravenous administration of NaBu liposomes and oral delivery of NaBu tablets could effectively inhibit the proliferation of F. nucleatum and significantly improve the therapeutic efficacy of oxaliplatin in mice with subcutaneous colorectal tumors, orthotopic colorectal tumors and even spontaneously formed colorectal tumors. Thus, our work provides a simple but effective formulation of NaBu to relieve F. nucleatum induced chemoresistance, exhibiting ideal clinical application prospects.
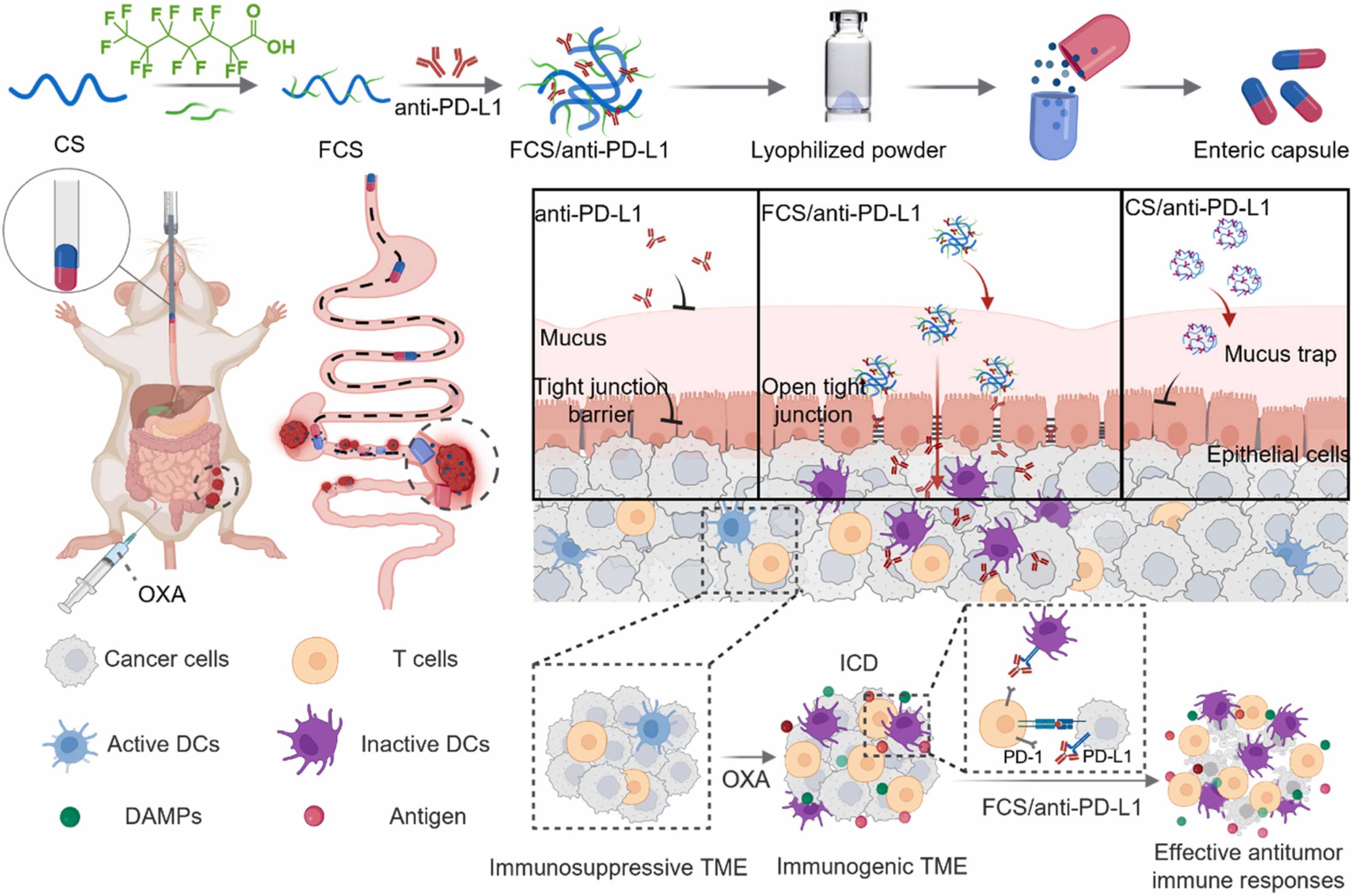
Oral delivery of anti-PD-L1 antibody for cancer immunotherapy against orthotopic colorectal tumors
Linfu Chen#, Lin Zhang#, Rui Zhao, Jingjing Shen, Yingyao Wang, Jiafei Zhu, Huapan Fang, Nanhui Liu, Cheng Wang, Ting Wei, Yu Chai, Maoyi Li, Chenghao Wu, Qian Chen*, Zhuang Liu* (# equal contribution, * corresponding author)
Nano today 2023 Q1IF=13.2
Despite the approval of immune checkpoint blockade (ICB) for colorectal cancer treatment, its objective response rate remains to be improved, especially for non-immunogenic tumors. Moreover, systemically administrated ICB antibodies is usually associated with immune-related adverse effects (irAEs). Herein, we report novel oral administration of anti-programmed cell death protein L1 (anti-PD-L1) complexes for immunotherapy against colorectal tumors. Antibodies could form nanocomplexes with fluorocarbon modified chitosan (FCS) to acquire greatly improved transmucosal penetration ability, owing to the ability of FCS to reduce mucus binding and temporary open tight junctions between epithelial cells. As evidenced in orthotopic colorectal tumors on mice, orally administrated FCS/anti-PD-L1 nanocomplexes exhibited greatly improved intestinal permeability and significant intertumoral penetration, and if combined with oxaliplatin (OXA) chemotherapy to induce an immunogenic tumor phenotype, could finally inhibit the growth of tumors and prolong the animal survival. Notably, compared to systematic injection of anti-PD-L1, oral administration of FCS/anti-PD-L1 achieved comparable therapeutic efficacy with five-fold dosage, and exhibited reduced tendency of systemic irAEs as exhibited by the decreased percentage of Th17 cells in lymph nodes. Our work here provides a simple but effective strategy for immunotherapy by oral administration of ICB antibodies, achieving strong antitumor immune responses without the concern of systemic irAEs.
Oral delivery of anti-PD-L1 antibody for cancer immunotherapy against orthotopic colorectal tumors
Linfu Chen#, Lin Zhang#, Rui Zhao, Jingjing Shen, Yingyao Wang, Jiafei Zhu, Huapan Fang, Nanhui Liu, Cheng Wang, Ting Wei, Yu Chai, Maoyi Li, Chenghao Wu, Qian Chen*, Zhuang Liu* (# equal contribution, * corresponding author)
Nano today 2023 Q1IF=13.2
Despite the approval of immune checkpoint blockade (ICB) for colorectal cancer treatment, its objective response rate remains to be improved, especially for non-immunogenic tumors. Moreover, systemically administrated ICB antibodies is usually associated with immune-related adverse effects (irAEs). Herein, we report novel oral administration of anti-programmed cell death protein L1 (anti-PD-L1) complexes for immunotherapy against colorectal tumors. Antibodies could form nanocomplexes with fluorocarbon modified chitosan (FCS) to acquire greatly improved transmucosal penetration ability, owing to the ability of FCS to reduce mucus binding and temporary open tight junctions between epithelial cells. As evidenced in orthotopic colorectal tumors on mice, orally administrated FCS/anti-PD-L1 nanocomplexes exhibited greatly improved intestinal permeability and significant intertumoral penetration, and if combined with oxaliplatin (OXA) chemotherapy to induce an immunogenic tumor phenotype, could finally inhibit the growth of tumors and prolong the animal survival. Notably, compared to systematic injection of anti-PD-L1, oral administration of FCS/anti-PD-L1 achieved comparable therapeutic efficacy with five-fold dosage, and exhibited reduced tendency of systemic irAEs as exhibited by the decreased percentage of Th17 cells in lymph nodes. Our work here provides a simple but effective strategy for immunotherapy by oral administration of ICB antibodies, achieving strong antitumor immune responses without the concern of systemic irAEs.